Background: Combining oral ABL-targeted tyrosine kinase inhibitors (TKIs) with (w/) multi-agent chemotherapy has improved the long-term disease-free survival of adults w/ Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL). However, Ph+ ALL occurs more commonly in older adults, and toxicities of multi-agent chemotherapy, including sequelae of prolonged myelosuppression, are amplified in this population. Others have previously reported rates of morphologic complete response (mCR) approaching 100% among adults w/ Ph+ ALL treated w/ corticosteroids (CS) and dasatinib (DAS) alone as induction therapy, but w/ low rates of minimal residual disease (MRD) negativity by flow cytometry (FACS) and BCR-ABL1 PCR (complete molecular response, CMR) and high rates of relapse in the absence of further consolidation.
The bispecific T-cell engager blinatumomab (BLIN) has considerable efficacy in clearing MRD in patients (pts) w/ Ph- B-cell ALL. We have previously reported our institutional experience combining ABL TKIs w/ BLIN in pts w/ Ph+ ALL and MRD, including encouraging safety data and high rates of MRD eradication (King/Geyer et al., Leuk Res, 2019). The ongoing D-ALBA study (GIMEMA LAL2116) is also investigating BLIN + DAS consolidation following 12 weeks of induction (prednisone + DAS followed by DAS), w/out protocol-specified maintenance, and has demonstrated preliminary evidence of efficacy (Chiaretti et al., ASH Meeting, 2019). As such, we designed a phase II study of BLIN as part of a chemotherapy sparing strategy in pts w/ Ph+ ALL (BLISSPHALL), introducing BLIN as early as 6 weeks into treatment for pts in morphologic CR, w/ aim of enhancing early MRD negativity and suppressing resistant clones early in disease course.
Study design and methods: Our institution is leading a phase II trial of TKI + BLIN consolidation and maintenance in adults w/ newly-diagnosed Ph+ ALL, w/ potential multicenter expansion (NCT04329325). Pts are eligible if they are ≥18 years-old w/ Ph+ ALL confirmed by cytogenetic or molecular studies, ECOG performance status 0-2, w/out prior therapy for ALL beyond CS, hydroxyurea, or intrathecal chemotherapy, w/out known active extramedullary disease and/or CNS-3 disease, and w/ appropriate organ function.
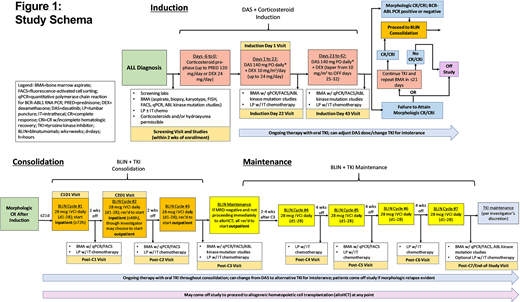
See Figure 1: pts will receive a CS pre-phase (days [d] -6 - 0) followed by modified GIMEMA LAL1205 induction (dexamethasone [DEX] 10 mg/m2 [max 24 mg/d], d1-24, tapered off d25-32) + DAS 140 mg/d (dose adjustments or TKI change permitted per protocol) w/ intrathecal methotrexate (IT MTX) d22, 43 and bone marrow (BM) assessments including FACS and BCR-ABL1 PCR. Pts in mCR on d43 (or optional reassessment ≤ 3 weeks later) will be eligible to proceed to consolidation w/ 3 cycles (C) BLIN 28 mcg/d IVCI, d1-28, concurrent w/ TKI, w/ 14d off BLIN between cycles and BM MRD assessment/IT MTX after each cycle. C1 BLIN + TKI is required to begin inpatient x72 hours. TKI is given continuously including between BLIN cycles. Pts in CMR after consolidation may proceed to maintenance (C4-7 BLIN + TKI, 28d off between cycles). Pts may come off study to proceed to hematopoietic cell transplant (HCT) at any point, though it is recommended such pts receive ≥2C of BLIN + TKI.
The primary objective is to determine the proportion of evaluable pts achieving CMR by the end of consolidation (≤ 3C BLIN + TKI). Secondary objectives include safety/toxicity of BLIN + DAS, duration of CMR, incidence of relapse, event-free/overall survival. Exploratory objectives include safety/toxicity of non-DAS TKIs + BLIN, defining patterns/mechanisms of resistance to BLIN+TKI (including ABL kinase mutations), and outcomes among pts not undergoing HCT.
The trial utilizes a Simon's minimax two-stage design; 20% CMR rate is considered not promising, a 50% CMR rate is considered promising, and probabilities of type I/II error are set at 0.10/0.10. If ≥ 3 of the first 10 pts achieve CMR we will continue enrollment to max 17 pts. If ≥ 6 pts achieve CMR, then BLIN + TKI will be considered promising for further investigation.
The investigators are hopeful this study will add to the currently limited prospective data supporting TKI + BLIN consolidation/maintenance for pts w/ Ph+ ALL and efforts to develop chemotherapy-sparing and immunotherapeutic strategies for older pts w/ ALL.
Geyer:Amgen: Research Funding. King:Abbvie: Other: advisory board. Park:Novartis: Consultancy; Genentech/Roche: Research Funding; Intellia: Consultancy; Artiva: Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Research Funding; Takeda: Consultancy, Research Funding; Servier: Consultancy, Research Funding; AstraZeneca: Consultancy; Allogene: Consultancy; Incyte: Consultancy, Research Funding; Kite: Consultancy, Research Funding; Juno Therapeutics: Research Funding; GSK: Consultancy; Autolus: Consultancy, Research Funding; Minverva: Consultancy; Amgen: Consultancy, Research Funding.
Blinatumomab is not approved to be given in combination with ABL tyrosine kinase inhibitors for treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia.
Author notes
Asterisk with author names denotes non-ASH members.